Currently Empty: RM0.00
Can a small organ quietly keep your blood pH steady while life, diet and Malaysia’s heat push fluid balance around?
They protect acid-base balance by sending acid into urine and keeping bicarbonate in blood. This constant work holds plasma near an ideal pH of about 7.40, with H+ around 40 nmol/L, and keeps bicarbonate close to 25–27 mmol/L.
The process starts at filtration and finishes in tiny nephron segments, where cells trade ions so urine can be much more acidic than plasma.
Normal urine pH ranges from 4 to 8 and daily volume averages ~1.5 liters, changing with fluid intake, exercise and hot weather in Malaysia. For friendly, local guidance, readers may contact Wellness Concept on WhatsApp at +60123822655 during business hours: Mon–Fri 9:30 am–6:30 pm and Sat–Sun 10 am–5 pm.
Key Takeaways
- Kidneys keep acid-base balance by excreting acid and reclaiming bicarbonate.
- Blood pH stays near 7.40 while urine pH varies widely to remove excess H+.
- CO2 and HCO3 interact with renal processes to stabilize plasma concentration.
- Urine volume and acidity shift with fluid intake, activity and Malaysia’s climate.
- Early changes often appear in urine and can signal wider body imbalance.
- Wellness Concept offers Malaysia-based support via WhatsApp at +60123822655.
Acid-base balance, hydrogen ions, and why kidneys matter now
A steady plasma pH is vital; small shifts would impair cell function across the body. Today, blood H+ stays tightly held at about 37–43 nmol/L, which corresponds to pH 7.37–7.43.
Blood pH in the present context: tight control around 7.37-7.43
Respiratory CO2 adjustments act fast, while renal processes fine-tune buffers and long-term balance. The organs adjust excretion and reabsorption so plasma concentration of bicarbonate and other bases stays stable.
Search intent decoded: what readers in Malaysia will learn from this Ultimate Guide
This section explains how urine chemistry mirrors systemic balance. Acidity or alkalinity shifts with diet, hydration, tropical heat and exertion.
- Key facts: blood pH range, renal excretion of H+, and ammonia production as needed.
- How much the system can change daily to protect steady blood chemistry.
- Practical cues: when urine clues suggest adjusting diet or fluid, and when to seek help.
| Measure | Normal range | What adjusts it | Clinical clue |
|---|---|---|---|
| Blood pH | 7.37–7.43 | Respiration, renal reabsorption | Stable H+ ~37–43 nmol/L |
| Urine pH | 4–8 | Diet, fluid, tubule handling | Acidic after high protein; alkaline with vegetables |
| Renal action | Variable | H+ excretion, NH3 production | Increased excretion when acid load rises |
For tailored advice in Malaysia, readers can message Wellness Concept on WhatsApp at +60123822655 (Mon–Fri 9:30 am–6:30 pm; Sat–Sun 10 am–5 pm).
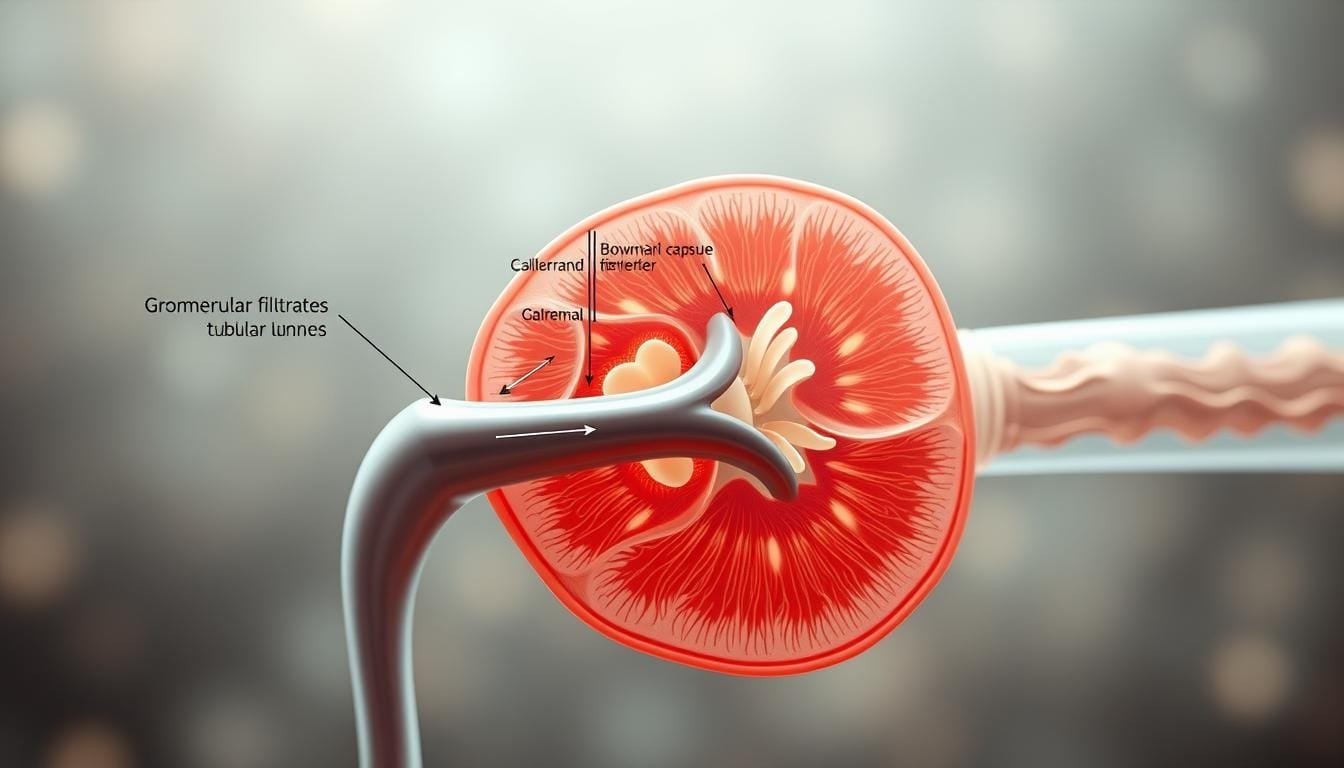
What does the kidney do with hydrogen ions?
Filtration at the glomerulus hands plasma to the tubule, where selective ion handling sets acid-base balance.
From glomerulus to tubule: where filtration ends and regulation begins
Plasma is filtered; most bicarbonate is reclaimed before filtrate leaves the proximal tubule. Tubular cells use enzymes and gradients to recover base and move acid into tubular fluid.
Proximal tubule chemistry and bicarbonate recovery
Carbonic anhydrase catalyzes CO2 + H2O → carbonic acid, which splits to H+ and bicarbonate. Secreted H+ combines with filtered bicarbonate to form CO2 and water, letting CO2 diffuse back into cells and restore bicarbonate to blood.
Distal secretion, buffers, and ammonia
Distal segments actively secrete H+. Phosphate in tubular fluid buffers much of that H+, producing titratable acidity of ~20–40 mmol/day.
Tubular cells also make ammonia from glutamine. NH3 binds H+ to form NH4+, trapping acid for excretion and boosting total acid removal.
Total handling and clinical note
Daily H+ handling averages 50–100 mmol and rises with higher acid loads. This system lets urine become very acidic or near neutral to keep plasma bicarbonate near 25–27 mmol/L.
For tailored, Malaysia-based advice about urine findings or acid-base balance, message Wellness Concept on WhatsApp at +60123822655 (Mon–Fri 9:30 am–6:30 pm; Sat–Sun 10 am–5 pm).
Why this matters for your health: urine pH, diet, and clinical clues
Daily urine pH gives simple clues about diet, hydration and metabolic balance.
Urine pH range and what shifts it
Normal urine pH spans roughly 4–8. Higher protein intake raises acid production and shifts chemistry toward lower pH.
Fluid intake, sweating in Malaysia’s heat, and metabolic state also change urine volume and concentration.
Reading the signals
Bicarbonate may appear in urine once plasma HCO3 exceeds about 27 mmol/L. That loss can signal a transient rise in base load.
Ammonium excretion rises when urine becomes more acidic. Titratable acidity—mainly phosphate buffering—usually totals about 20–40 mmol per day.
When to seek tailored advice in Malaysia
Consistent shifts in urine pH, unexpected bicarbonate in a sample, or changes in volume merit friendly professional review.
For personalized guidance on diet, hydration and interpreting urine results, message Wellness Concept on WhatsApp at +60123822655 (Mon–Fri 9:30 am–6:30 pm; Sat–Sun 10 am–5 pm).
Conclusion
Everyday urine clues reflect how cells and the tubule steady plasma pH. This balance relies on coupled removal of hydrogen and recovery of bicarbonate while CO2 is handled across tissues.
Key takeaways: kidneys keep blood near pH 7.40 by matching hydrogen removal in urine with HCO3 reabsorption. Total acid disposal commonly totals 50–100 mmol per day. Urine pH normally spans 4–8 and shifts with diet, fluid and ammonia-driven buffering.
Next steps: stay hydrated, watch dietary patterns, and seek review when urine changes persist. For friendly, Malaysia-based advice, message Wellness Concept on WhatsApp at +60123822655 (Mon–Fri 9:30 am–6:30 pm; Sat–Sun 10 am–5 pm).
FAQ
How do kidneys help keep blood pH around 7.37–7.43?
They fine-tune acid-base balance by reabsorbing filtered bicarbonate, secreting hydrogen ions in tubular fluid, and generating new bicarbonate through ammonia production. Tubular cells use carbonic anhydrase to convert CO2 and H2O into carbonic acid, which yields bicarbonate ions that return to plasma, keeping extracellular pH stable.
Where does filtration end and regulation begin along the nephron?
Filtration at the glomerulus delivers plasma to nephrons, then regulation occurs along tubules. The proximal tubule reclaims most bicarbonate and solutes. The loop of Henle and distal nephron adjust ion transport, while collecting ducts perform final H+ secretion and ammonia trapping to control urinary acidity.
What role does the proximal tubule play in bicarbonate recovery?
Proximal tubular cells use carbonic anhydrase to convert luminal CO2 and H2O into carbonic acid, then into H+ and bicarbonate. H+ is secreted back into tubular fluid in exchange for sodium, while bicarbonate is reabsorbed into blood. This preserves about 80–90% of filtered bicarbonate.
How is plasma bicarbonate kept near 25–27 mmol/L?
Reabsorption of filtered bicarbonate and generation of new bicarbonate by the distal nephron balance daily acid production. When plasma concentration falls, renal H+ secretion increases and more NH4+ is produced, raising reclaimed bicarbonate and restoring levels toward the normal range.
What happens in the distal nephron to handle acid?
Principal and intercalated cells actively secrete H+ using H+-ATPase and H+/K+-ATPase. Phosphate acts as a titratable buffer in tubular fluid, accepting H+ to form H2PO4−. These processes create titratable acidity and help eliminate fixed acids while conserving bicarbonate.
How does ammonia production assist acid excretion?
Tubular cells metabolize glutamine to produce NH3, which diffuses into tubular fluid and binds H+ to form NH4+. Trapped NH4+ is excreted in urine, allowing net removal of acid and generation of new bicarbonate for plasma, an essential mechanism during higher acid loads.
How much net H+ do kidneys handle per day?
Total renal acid handling varies but commonly ranges from about 50 to 100 mmol per day. This amount shifts with dietary acid load, metabolic state, and kidney health, influencing rates of bicarbonate regeneration, ammonium excretion, and titratable acidity.
What urine pH range is normal and what affects it?
Urine pH typically spans roughly 4 to 8. Dietary protein, hydration, and systemic acid-base status shift pH: high protein and metabolic acidosis lower it, while plant-rich diets or alkalosis raise it. Urinary buffers like phosphate and ammonium determine final acidity.
Which urinary measurements provide clinical clues about acid-base status?
Urine bicarbonate appears only when plasma bicarbonate is very high. Ammonium excretion and titratable acidity indicate renal acid excretion capacity. Low NH4+ with acidosis suggests impaired tubular acid handling; high titratable acidity shows active phosphate buffering.
When should someone seek tailored advice in Malaysia?
They should consult a clinician when persistent abnormal blood pH, unexplained fatigue, recurrent kidney stones, or abnormal urine tests appear. For local support, contact Wellness Concept on WhatsApp at +60123822655 for guidance and referral to nephrology services.